EXTENSIVE CLINICAL AND PRECLINICAL PROGRAMS
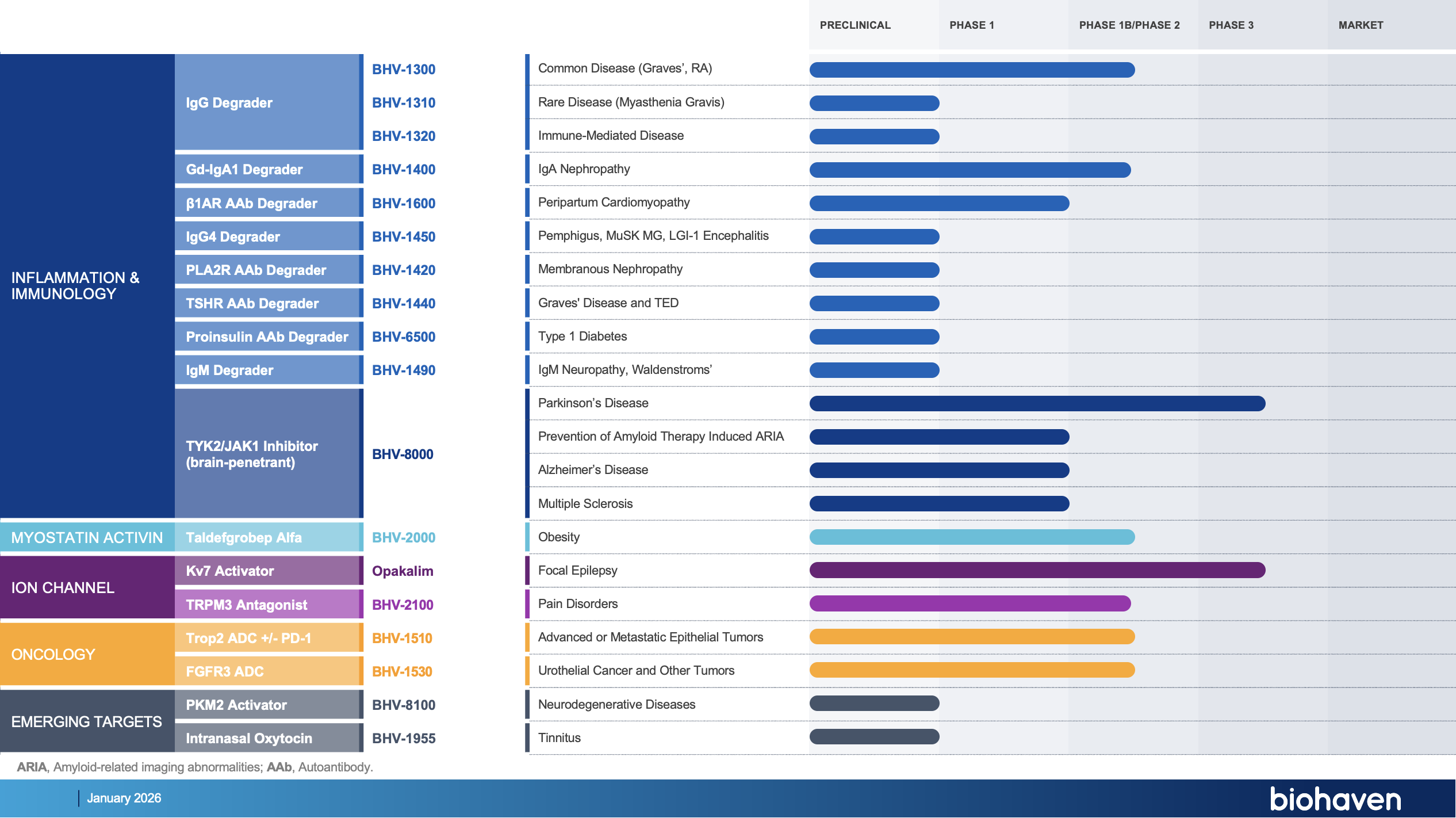
Our portfolio includes Kv7 ion channel modulation for epilepsy and mood disorders; extracellular protein degradation for immunological diseases; TRPM3 antagonism for pain disorders; TYK2/JAK1 inhibition for neuroinflammatory disorders; myostatin inhibition for neuromuscular and metabolic diseases, including SMA and obesity; antibody recruiting bispecific molecules; and antibody drug conjugates for cancer.
EXPLORE OUR INTERACTIVE PIPELINE BELOW FOR MORE DETAILS
Spinal Muscular Atrophy
BHV-2000
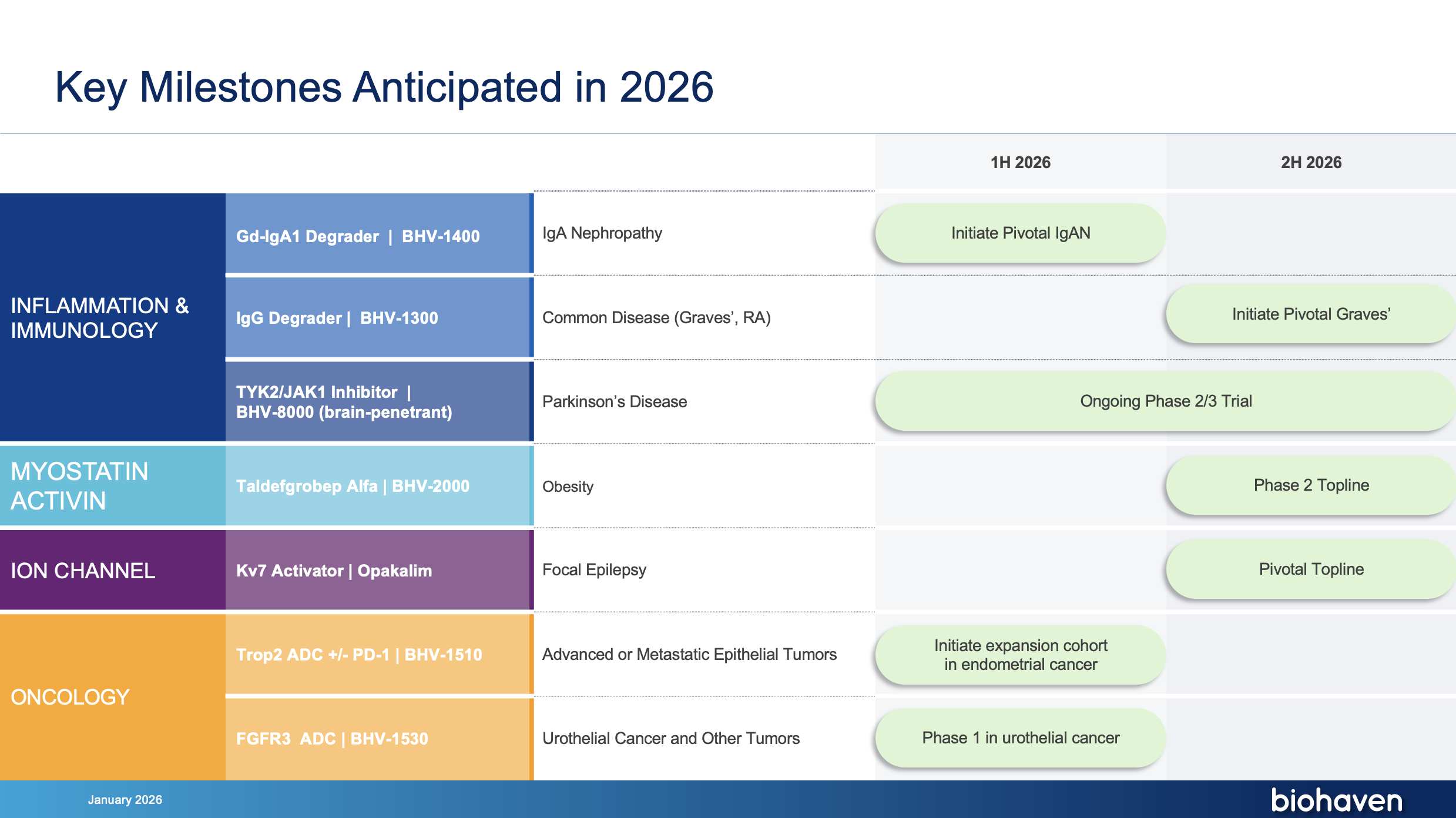
Obesity
BHV-2000
Focal Epilepsy
Opakalim (BHV-7000)
Generalized Epilepsy
Opakalim (BHV-7000)
Major Depressive Disorder
Opakalim (BHV-7000)
Pain Disorders
BHV-2100
TYK2/JAK1 Inhibitor
(brain-penetrant)
Parkinson’s Disease
BHV-8000
Prevention of Amyloid Therapy Induced ARIA
BHV-8000
Alzheimer’s Disease
BHV-8000
Multiple Sclerosis
BHV-8000
IgG Degrader
Common Disease (Graves’, RA)
BHV-1300
Rare Disease (Myasthenia Gravis)
BHV-1310
Gd-IgA1 TRAP Degrader
IgA Nephropathy
BHV-1400
β1AR Autoantibody
Degrader
Peripartum Cardiomyopathy
BHV-1600
Trop2 ADC +/- PD1
Advanced or Metastatic Epithelial Tumors
BHV-1510
FGFR3 ADC
Urothelial Cancer & Other Tumors
BHV-1530
CD30 ADC
Hodgkin Lymphoma
BHV-1500
Undisclosed Targets
Merus and GeneQuantum Collaborations